Researchers at The Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai have demonstrated the potential for a new combination therapy to improve outcomes for patients with myelofibrosis, a rare and aggressive blood cancer.
The phase 3 trial, published this month in Nature Medicine, represents the first randomized study of JAK inhibitor-based combination therapy in treatment-naive myelofibrosis patients, signaling a major advancement in disease management.
The study, led by John Mascarenhas, MD, Director of the Center of Excellence for Blood Cancers and Myeloid Disorders at Mount Sinai, is the first major study to test a combination of two medicines to treat myelofibrosis, instead of using one at a time. The trial evaluated the efficacy and safety of pelabresib plus ruxolitinib in patients newly diagnosed with myelofibrosis. The findings suggest that combining these targeted therapies results in deeper clinical responses, with the potential to modify the disease course and improve overall survival.
“We are hopeful that this study will mark the beginning of a new era in myelofibrosis treatment,” said Dr. Mascarenhas. “For too long, patients have relied on single-agent JAK inhibitors that—while beneficial—fail to modify the disease course for most individuals. Our findings highlight the power of rationally designed combination therapies to deliver deeper and more durable responses.”
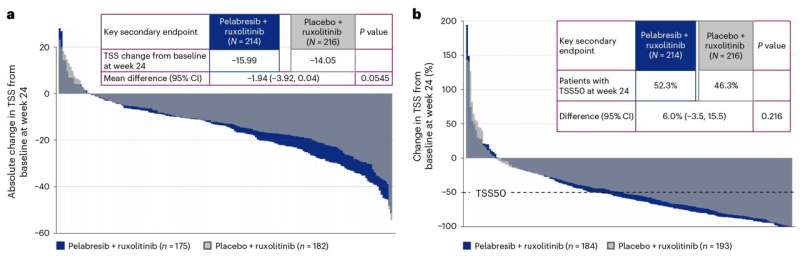
According to the American Association for Cancer Research, there are an estimated 13,000 people living with myelofibrosis in the United States. Myelofibrosis patients who fail monotherapy treatments typically experience poor outcomes within two to three years. This study suggests that combination therapy could lead to longer-lasting responses, improved disease control, and potentially extended survival.
The data from this trial could lead to regulatory approval of combination therapy, establishing a new standard of care for early myelofibrosis management. The study’s robust correlative analyses further support the potential of this combination to alter disease progression, offering hope for patients who currently face a median survival of just five years.
Next, the research team will continue to monitor patients for long-term survival outcomes. Additional data from the study will be presented at the European Hematology Association meeting in June 2025 and at the American Society of Hematology conference in December, providing further insights into the therapy’s long-term benefits.
The study was conducted across 80 medical centers worldwide.
More information:
Raajit K. Rampal et al, Pelabresib plus ruxolitinib for JAK inhibitor-naive myelofibrosis: a randomized phase 3 trial, Nature Medicine (2025). DOI: 10.1038/s41591-025-03572-3
The Mount Sinai Hospital
Citation:
JAK inhibitor combo therapy shows promise for myelofibrosis treatment in phase 3 trial (2025, March 20)
retrieved 20 March 2025
from https://medicalxpress.com/news/2025-03-jak-inhibitor-combo-therapy-myelofibrosis.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.